B.1.1.
|

|
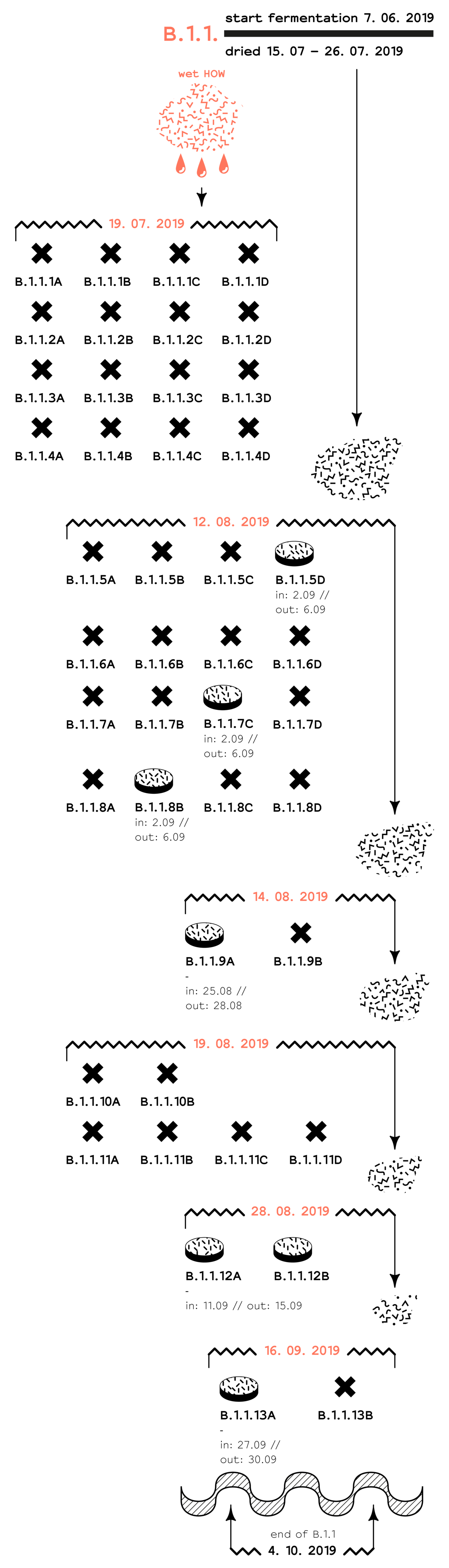
Experiment 19. 07. 2019: 1st pH experiment⌕ Can wet and unsterilised HOW be used as a substrate for mycelium forming?
Fermented HOW, either dried or wet is an acidic substrate and many fungi have difficulties growing in such environments. In this experiment we tried to see if raising the pH of the HOW would change the mycelium forming process, by comparison with what we've noticed before. Additionally, we wanted to see if the ganoderma mycelium spawn can be the dominant organism that grows when HOW is not dried (hence plenty of other organisms are active in it), but the pH is basic.16 medium Petri dishes were prepared, all with the same contents, after homogeneously mixing a total of: 100g ganoderma mycelium spawn + 262g wet HOW (original pH 4.5) + 4g sodium bicarbonate (NaHCO3). Resulting pH = 6.39 → near the optimal pH environment in which ganoderma grows well. All dishes were left to grow at 25 – 30 degrees Celsius. After 10 days no growth could be seen and the samples were all thrown away. Two reasons might have caused the failure:
|

|
Experiment 12. 08. 2019: do-over of the 1st pH experiment⌕ Does raising the pH of fermented HOW influence the course of mycelium forming?In this experiment we tried to see if raising the pH of the HOW would change the mycelium forming process, by comparison with what we've noticed before.
Resulting pH = 5.86. The above mixture was divided between the 16 dishes. Half of the dishes were prepared very wet, and half moist, as provided below: All dishes were left to grow at 25 – 30 degrees Celsius. Only 3 of all were successful; these were dried and incubated at 65 degrees Celsius. The results of this experiment were inconclusive. 
|

|
Experiment 14. 08. 2019: 2nd pH experiment⌕ Does raising the pH of fermented HOW influence the course of mycelium forming?The pH of fermented HOW is acidic. After previous attempts in which HOW's pH was raised using sodium bicarbonate, we started to consider that the composition of the dried HOW could also play a role in the mycelium forming stage. This is because in previous pH raising attempts the samples died seemingly random.In all experiments so far, B.0.1 performed better than B.1.1. This experiment compares the mycelium forming stage when the pH of both these batches is brought up with sodium bicarbonate. This is done to find out if the composition of dried HOW matters. 4 medium Petri dishes were prepared as follows: 
All dishes were left to grow at 25 – 30 degrees Celsius. As of both batch sets (B.0.1 and B.1.1) only the dishes treated with sodium bicarbonate failed, we conclude that the ganoderma mycelium cannot feed on sodium bicarbonate and that in this particular case the composition of HOW doesn't matter in the mycelium forming stage. 
|

|
Experiment 19. 08. 2019: 2nd colouring attempt⌕ Can the HOW material be coloured?
Before this experiment, a very small scale colouring experiment was done with red silk dye.While previously small Petri dishes were used, now 2 medium Petri dishes are prepared to see if the the mycelium will form and if the colour will not be decomposed by the fungal enzymes: 

In-between pH experiment⌕ Does raising the pH of fermented HOW influence the course of mycelium forming?In the previous pH experiments, sodium bicarbonate seemed to have influenced mycelium forming negatively. In this attempt we tried to use a readily available household item that has a basic composition: dishwashing liquid.4 medium Petri dishes were prepared as follows: 
|

|
Experiment 28. 08. 2019: a repetition experiment⌕ Will repeating the same experiment lead the same results?
In the weeks leading up to this experiment, HOW samples have not been developing very well. This experiment focuses on verifying if repeating the conditions of successful samples will give the same results. Additionally, mycelium forming
direction and structure are tested. 

The batch samples from B.0.1 and B.1.1 grew the same as before. B.3.1 and B.3.2 were newly introduced and behaved differently in the repetition conditions. This could have to do with the composition of the dried HOW. Mixed samples became slightly curved towards the top, while the layered samples formed flat. A cause of this could have been that mycelium always forms its net upwards, thereby constructing a denser mass and tension in the upper part of the dish. |

|
Experiment 16. 09. 2019: colouring attempt 03⌕ Can the HOW material be coloured?
This experiment focuses on testing if different colour dyes preserve during the mycelium forming stage, and if colour dye hinders mycelium forming when the HOW substrate is present. 15 medium Petri dishes were prepared; 10 dishes containing HOW substrates from all batches laying around at the time, and 5 control dishes. The red and blue silk dyes were poured on top of the dishes after the mycelium spawn and the HOW were mixed. As yellow dye, turmeric powder was mixed homogeneously in the mycelium control dishes. 





The blue silk dye was decomposed during mycelium forming, the colour dissapeared until the end of the process. Of the 3 yellow dye control dishes, the C sample didn't grow. This experiment concluded that colouring the HOW material is possible but the exact conditions in which this must happen are yet to be researched. |

|